Professor Sir Nicholas J White

Podcast interview
Improving the treatment of infectious diseases
With nearly 50 years in malaria research and more recent focus on COVID-19, research at MORU led to more effective treatments. In COVID-19, trials debunked drugs like ivermectin or favipiravir, but validated remdesivir, molnupiravir and protease inhibitors. As malaria faces drug resistance, triple therapies offer hope. MORU research aims for tangible health impacts, with an approach applicable to other infectious diseases.
Research groups
Colleges
Nicholas White
FRS
Professor of Tropical Medicine
- Professor at Mahidol University in Thailand
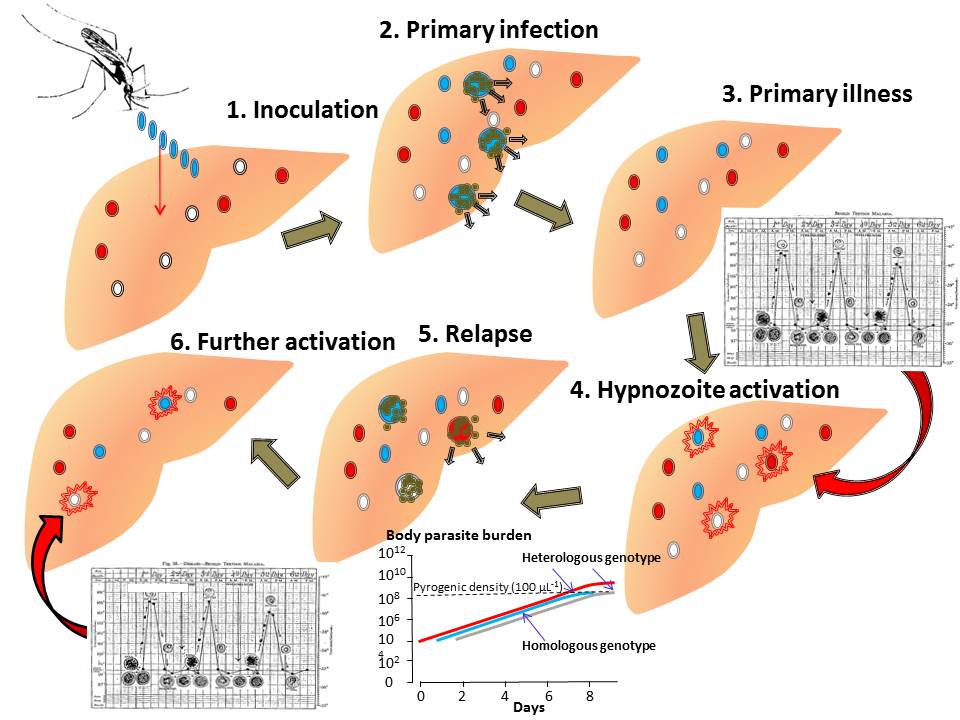
Professor White’s diverse interests include the epidemiology, pathophysiology and management of uncomplicated and severe malaria, meliodosis, enteric fever, tetanus, dengue haemorrhagic fever, Japanese encephalitis and tuberculosis. His particular interests at present include the pathophysiology and treatment of severe malaria, the prevention of antimalarial drug resistance using artemisinin-based combinations. and the biology of relapse in vivax malaria.
Professor Sir Nick White passed away on the 1st February 2026
Recent publications
-
Accurate measurement of viral clearance in early phase antiviral studies in COVID-19.
Journal article
Wongnak P. et al, (2025), J Infect Dis
-
Assessing monoclonal antibodies for respiratory virus infections.
Journal article
White NJ. et al, (2025), Lancet, 406, 595 - 596
-
Microhaplotype deep sequencing assays to capture Plasmodium vivax infection lineages.
Journal article
Kleinecke M. et al, (2025), Nat Commun, 16
-
Does mass chloroquine treatment have any role in the elimination of Plasmodium vivax ?
Journal article
White NJ. et al, (2025), Malar J, 24
-
Characterising viral clearance kinetics in acute influenza
Preprint
Wongnak P. et al, (2025)